Most people assume that before a generic drug hits the market, it must be tested on humans to prove it works the same as the brand-name version. That’s not always true. The FDA lets drugmakers skip human studies entirely - if they meet strict scientific criteria. This is called a bioequivalence waiver, or biowaiver. It’s not a loophole. It’s a science-backed shortcut that saves time, money, and avoids unnecessary human testing.
Why the FDA Allows Biowaivers
The FDA doesn’t require in vivo studies when in vitro data is more accurate, sensitive, and reproducible. That’s written into 21 CFR 320.24(a). For certain drugs, how quickly they dissolve in a test tube predicts exactly how they’ll behave in your body. If the dissolution profile matches the brand drug - down to the minute - then human trials become redundant. This isn’t theoretical. Between 2012 and 2016, about 15% of generic drug applications included biowaiver requests. Of those, 78% got approved when the science was solid. That’s a high success rate for a process that avoids spending $250,000 to $500,000 per study and cutting 6 to 12 months off the approval timeline.Which Drugs Qualify?
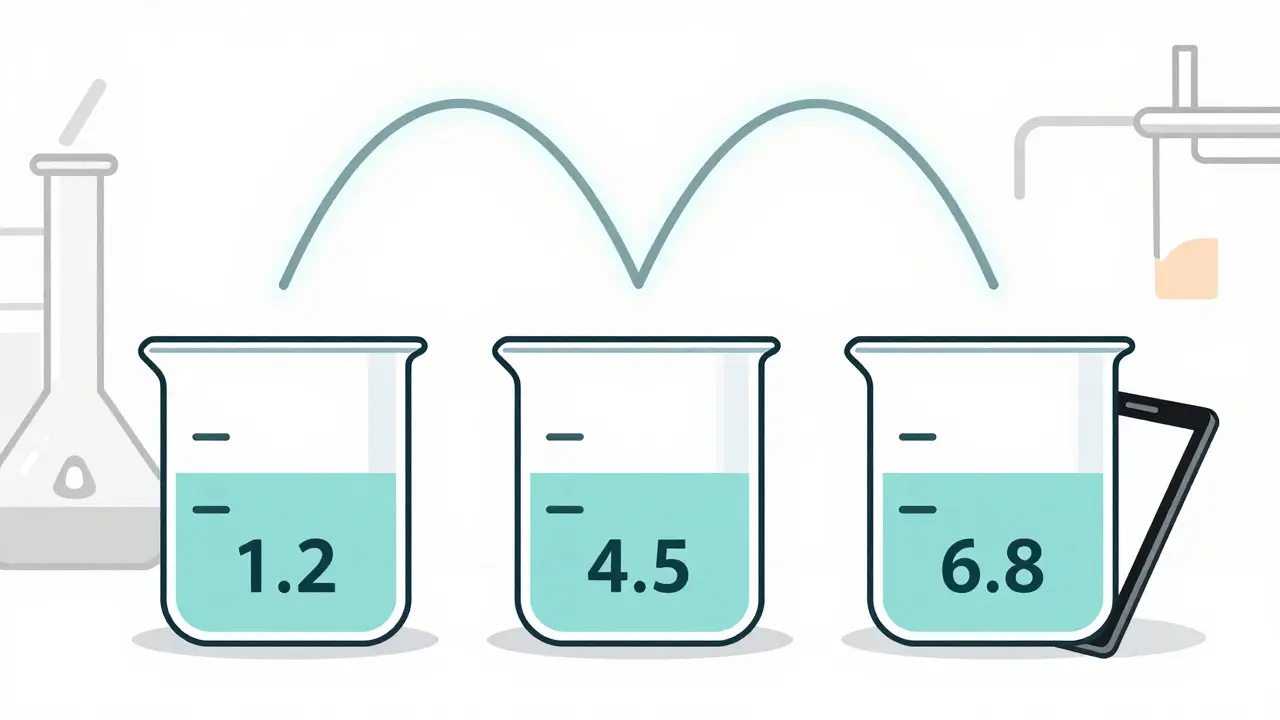
Not every pill can skip human testing. The FDA only allows biowaivers for immediate-release solid oral dosage forms - think tablets and capsules you swallow. The key is the drug’s classification under the Biopharmaceutics Classification System (BCS). There are four classes, but only two are eligible:- BCS Class I: High solubility, high permeability. These drugs dissolve easily and are absorbed completely. Examples include metoprolol, atenolol, and ciprofloxacin. For these, the FDA requires dissolution profiles to match the reference drug at pH 1.2, 4.5, and 6.8, with an f2 similarity factor of at least 50. That means the curves must be nearly identical across all time points.
- BCS Class III: High solubility, low permeability. These drugs dissolve well but don’t cross cell membranes easily. Examples include acyclovir and aliskiren. For these, the requirements are stricter: identical excipients, same proportions, and proof that absorption doesn’t depend on where in the gut the drug is released.
What the FDA Requires for Approval
Submitting a biowaiver isn’t just checking a box. You need hard data. The FDA demands:- Dissolution testing on at least 12 units of each formulation
- Sampling at 10, 15, 20, 30, 45, and 60 minutes
- Testing in three pH buffers: stomach (1.2), small intestine (4.5), and colon (6.8)
- A validated method that can detect even small formulation differences
Who Uses Biowaivers - and Why
Generic drugmakers are the biggest users. Teva and Mylan include biowaivers in 25-30% of their development pipelines. For them, it’s a cost-saver. One formulation scientist reported saving $4.2 million and gaining 8-10 months per product across 12 approved waivers. The impact is real. In fiscal year 2022, biowaivers appeared in 18% of ANDA submissions - up from 12% in 2018. IQVIA estimates this has accelerated generic approvals by 7.3 months per product, unlocking $1.2 billion in earlier market access annually. But it’s not easy. Smaller companies struggle. The process needs experts in dissolution method development, BCS classification, and regulatory writing. It takes 2-3 months just to develop a reliable dissolution method - and another 1-2 months to run comparative tests. Without that expertise, applications get rejected.Where the System Falls Short
Despite its success, the biowaiver system has gaps. Many companies report inconsistent decisions across FDA review divisions. A 2022 PhRMA survey found 42% of respondents saw uneven application of the rules. One regulatory affairs specialist noted that 3 out of 5 Class III waiver requests were rejected - even though they met all technical criteria. The biggest limitation? Modified-release products. If the pill is designed to release over 8 or 12 hours, biowaivers don’t apply. The FDA says current methods can’t predict in vivo performance for these. That’s 85% of complex generic drugs, according to a 2023 FDA committee report. Narrow therapeutic index drugs - like warfarin or levothyroxine - are also mostly excluded. Too risky. But there’s a pilot program underway to test biowaivers for certain antiepileptic drugs. That could change things.
The Future of Biowaivers
The FDA is expanding. Its 2023-2027 strategic plan aims to increase biowaiver opportunities by 25% by refining BCS criteria and improving in vitro-in vivo correlation models. A 2022 draft guidance proposed extending waivers to more Class III drugs. Evaluate Pharma predicts biowaivers will cover 25-30% of all ANDA submissions by 2027. The agency is investing $15 million annually through GDUFA to improve testing methods. That’s not just bureaucracy - it’s science catching up to real-world needs. As dissolution technology gets better, more drugs may qualify. But the core principle stays the same: if a test tube can tell you what a human body will do, why make people take a pill just to prove it?What This Means for Patients
You might never know a drug got approved via biowaiver. But you’ll feel the impact. Faster approvals mean more affordable generics hit the market sooner. For people on chronic meds - diabetes, high blood pressure, epilepsy - that’s not just convenience. It’s access. The science behind biowaivers is sound. It’s not about cutting corners. It’s about using the right tool for the job. For drugs that dissolve and absorb predictably, human trials add cost and delay - without adding safety or efficacy data. The FDA’s system works because it’s grounded in biology, not bureaucracy. And as long as companies stick to the science, patients win.What is a bioequivalence waiver?
A bioequivalence waiver, or biowaiver, is when the FDA allows a drugmaker to skip human studies and use in vitro dissolution data instead to prove a generic drug performs the same as the brand-name version. This is only allowed for certain immediate-release solid oral drugs that meet strict BCS criteria.
Which drugs can get a biowaiver?
Only immediate-release solid oral dosage forms classified as BCS Class I (high solubility, high permeability) or BCS Class III (high solubility, low permeability). Drugs must dissolve rapidly and completely, and their dissolution profile must match the reference drug exactly under specific pH conditions.
How is bioequivalence proven without human studies?
Through comparative dissolution testing. The generic drug and the brand drug are tested side by side in buffers mimicking stomach and intestinal pH. The dissolution curves must be nearly identical, with an f2 similarity factor of at least 50. This proves the drug releases at the same rate and extent - which, for Class I and III drugs, reliably predicts how it will behave in the body.
Why are modified-release drugs excluded?
Because their absorption depends on how slowly they release the drug over time - which can vary based on stomach emptying, food, and other factors. Current in vitro methods can’t reliably predict those complex in vivo behaviors. The FDA says biowaivers aren’t scientifically valid for these products yet.
Do biowaivers compromise safety?
No. The FDA requires the same level of proof - just using a different method. For Class I drugs, over 95% of biowaiver approvals match the results of in vivo studies. The system only works because it’s backed by decades of pharmacokinetic data and strict testing standards. Skipping human trials doesn’t mean skipping science.





Mel MJPS
January 27, 2026 AT 16:19Finally, someone explains this without jargon overload. I’ve been on meds for 10 years and never knew my $4 generic was approved without a single human taking a pill. Wild.
Katie Mccreary
January 27, 2026 AT 23:5178% approval rate? That’s not science, that’s corporate lobbying dressed up as pharmacology. They’re cutting corners to profit. Period.
Anna Lou Chen
January 29, 2026 AT 14:47Ah yes, the BCS system - a beautiful reductionist fantasy where molecules are just soluble little ghosts floating through membranes. But the body isn’t a test tube, it’s a chaotic symphony of microbiota, pH fluctuations, and existential dread. You can’t quantify soul.
Kathy Scaman
January 29, 2026 AT 22:34My pharmacist said biowaivers are why my blood pressure med dropped from $200 to $8. I’m not mad. If a test tube can do it, let it.
Kevin Kennett
January 30, 2026 AT 01:34People act like this is some shady loophole but it’s not. The FDA’s got decades of data backing this. If your drug dissolves like the brand and you’re BCS I or III, you’re not skipping science - you’re doing it smarter. Stop fearing innovation.
Howard Esakov
January 30, 2026 AT 13:38Of course the FDA approves these - they’re just trying to keep the generics industry from collapsing under its own weight. Real science? Nah. Budget math with a lab coat. 😏
Rose Palmer
January 31, 2026 AT 17:37The regulatory framework governing biowaivers is grounded in robust pharmacokinetic principles, as codified under 21 CFR 320.24(a). The statistical rigor of the f2 similarity metric ensures comparability across formulations, and empirical validation from over 1,200 approved applications confirms its reliability. This is not anecdotal - it is evidence-based policy.
Mindee Coulter
February 2, 2026 AT 17:21So basically if your pill dissolves the same in a cup of acid as the brand you’re good to go? Cool. More cheap meds please
Irebami Soyinka
February 3, 2026 AT 03:52USA thinks it’s so smart with its test tubes and f2 factors but in Nigeria we still fight for basic meds. You all play with your pH buffers while people die waiting for $1 pills. This ain’t progress - it’s privilege with a lab report. 🤡
Lance Long
February 3, 2026 AT 04:24Let me tell you something - I used to work in a generic lab. We spent 3 months just perfecting one dissolution method. It’s not easy. And when the FDA rejects you? It’s not because they’re mean. It’s because they’ve seen too many bad methods fool everyone. This system is brutal… but it works. Trust the grind.
Bryan Fracchia
February 5, 2026 AT 00:27It’s beautiful, really. Science doesn’t need to replicate the body if it already understands how the pieces fit. Why make someone swallow a pill just to confirm what a machine can show? Less pain, more access. That’s not just smart - it’s humane.
fiona vaz
February 6, 2026 AT 02:44Class III drugs are tricky - even small changes in excipients can mess with absorption. That’s why the FDA demands identical formulations. One company got rejected because their filler had 0.2% more magnesium stearate. Tiny. Catastrophic.
Jess Bevis
February 6, 2026 AT 23:31My cousin’s in Nigeria. He takes generic antiretrovirals. If biowaivers help get these to places like that faster, then yeah - let the test tubes do the work.
Sue Latham
February 7, 2026 AT 06:43Oh please. If you think this is just about science, you’ve never seen a pharma exec’s bonus structure. They don’t care if it’s BCS I or IV - they care if it saves them $3M and gets them to market 6 months early. Call it what it is: profit-driven deregulation disguised as innovation.