Every year, hospitals in the U.S. and Canada scramble to find basic medicines that should be easy to get-antibiotics, anesthesia, chemotherapy drugs, even saltwater IV bags. These aren’t rare or experimental treatments. They’re the backbone of everyday care. And yet, they keep disappearing. The reason? Not bad luck. Not a sudden spike in demand. It’s a broken system built on cheap prices, overseas factories, and zero backup plans.
Manufacturing Failures Are the #1 Cause
Over 60% of all generic drug shortages trace back to problems at the manufacturing level. That’s not a small glitch. It’s a pattern. A single contaminated batch can shut down an entire production line for months. Equipment breaks down. Clean rooms fail inspections. Quality control lapses trigger FDA warnings. And when that happens, there’s often no one else ready to step in.
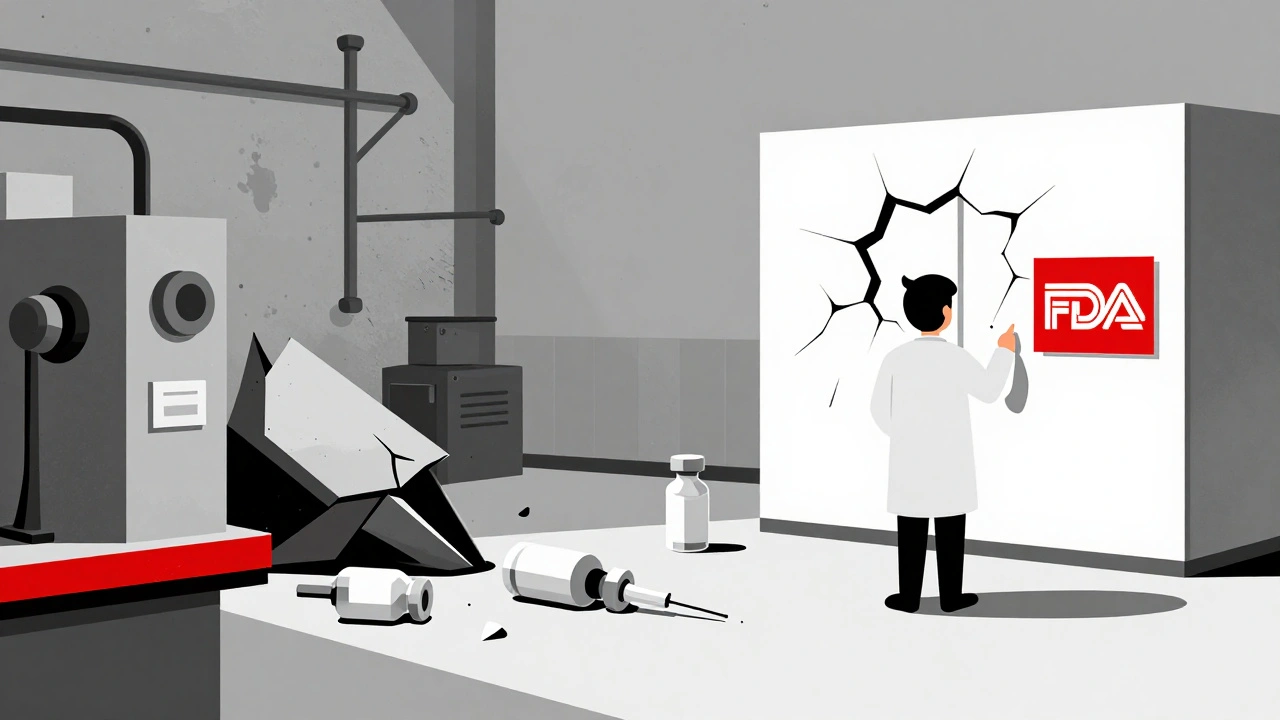
Take sterile injectables-the kind given directly into veins. These are among the most vulnerable. They require ultra-clean environments, complex machinery, and strict temperature controls. One FDA inspection in 2021 found a major facility had mold growing on walls, improperly sealed pipes, and untrained staff handling sterile products. The result? A 14-month halt in production of a key cancer drug. Patients had to wait. Doctors had to switch to less effective alternatives. Some treatments were delayed.
These aren’t rare events. They happen every few months. And because manufacturers run their plants at maximum capacity-with no extra machines, no spare parts, no buffer stock-they can’t absorb even a minor hiccup. There’s no safety net. Just one factory making one drug. If it goes down, the drug vanishes from shelves nationwide.
Most Drugs Are Made Overseas-And Only in Two Countries
Eighty percent of the active ingredients in generic drugs come from just two countries: China and India. That’s not a coincidence. It’s a business decision. Labor is cheaper. Regulations are looser. And companies can make more profit by cutting corners.
But this creates a massive single point of failure. If a flood hits a factory in Hyderabad. If an earthquake disrupts shipping in Shanghai. If a government suddenly restricts exports to protect its own population-boom. The entire U.S. supply chain stumbles. In 2020, when COVID-19 lockdowns hit India, dozens of essential medicines disappeared overnight. Antibiotics. Blood pressure pills. Even insulin. No one had a backup plan because no one expected to need one.
And it’s not just the ingredients. Finished pills, injections, and capsules are also mostly made overseas. The U.S. has lost most of its domestic manufacturing capacity over the past 30 years. Why build a $100 million clean room when you can buy the same product for half the price from a factory 8,000 miles away? The math makes sense-until it doesn’t.
There’s No Room for Error-Because There’s No Extra Capacity
Generic drugs are sold on razor-thin margins. Sometimes as low as 5% profit. That’s not enough to invest in backup equipment, redundant systems, or extra inventory. So manufacturers run their lines 24/7. They use every machine. They hire just enough staff. They keep minimal spare parts.
This is called ‘just-in-time’ manufacturing. It works great when everything runs smoothly. But when a machine breaks, or a worker gets sick, or a shipment gets stuck at customs, there’s no cushion. No spare capacity. No emergency stockpile.
Compare that to branded drugs. Companies like Pfizer or Merck keep extra inventory. They have multiple factories. They invest in redundancy because their profit margins are 30-40%. They can afford to be safe. Generic manufacturers can’t. And so, when something goes wrong, the entire system collapses.

One Company, One Drug-No Competition
One in five drug shortages involves a product made by only one manufacturer. That’s not a coincidence. It’s the result of years of price pressure.
When a generic drug first hits the market, multiple companies rush to produce it. Prices drop fast. Within a year, the cheapest bidder wins. The others either leave the market or switch to more profitable products. Soon, only one company is left making that drug.
Take the antibiotic vancomycin. For years, it was made by three companies. Then two dropped out. Then one. Now, if that last factory has a problem, millions of patients can’t get the drug they need to fight deadly infections. And because no one else is making it, there’s no competition to bring prices back up or incentivize someone else to enter the market.
Even when a drug is in short supply, manufacturers don’t rush to fill the gap. Why? Because the price is locked in. If they ramp up production, they’ll still get paid the same low rate. The cost of hiring workers, upgrading equipment, and passing inspections? That’s on them. The profit? Still tiny.
Pharmacy Benefit Managers Control the Market-and Make It Worse
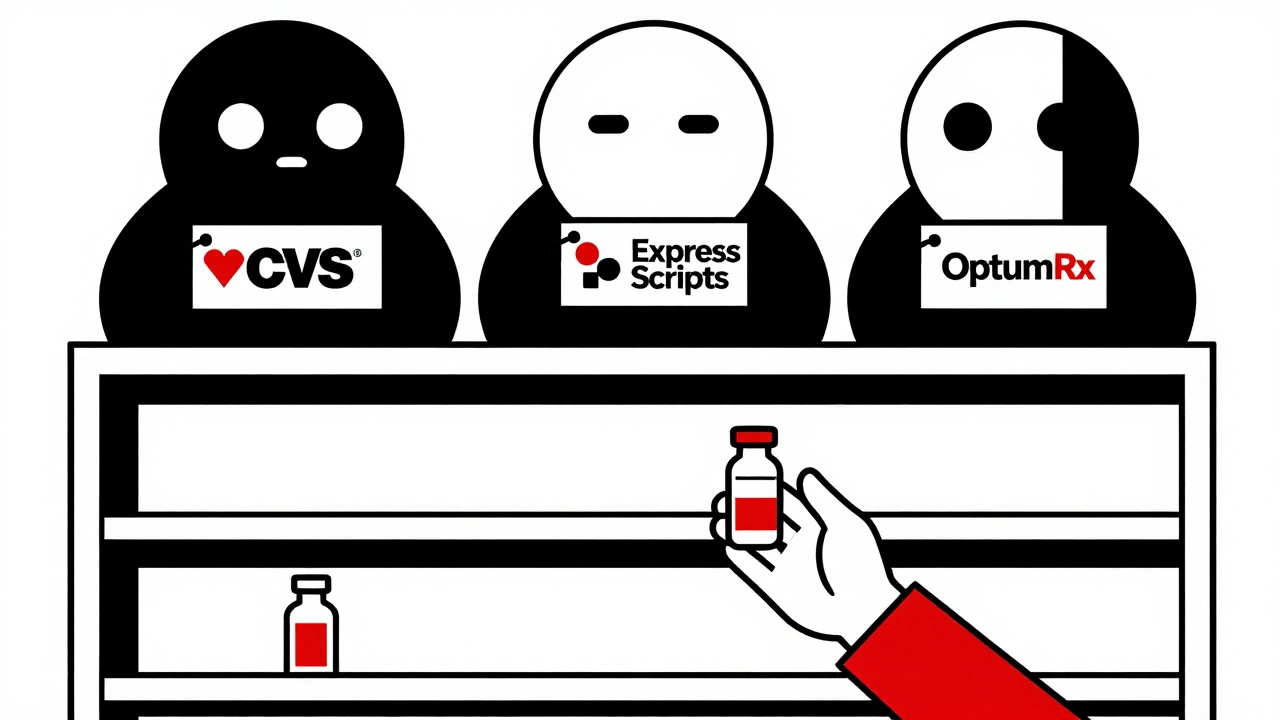
Three companies-CVS Caremark, Express Scripts, and OptumRx-control 85% of how prescription drugs are bought and paid for in the U.S. These are pharmacy benefit managers, or PBMs. They don’t make drugs. They don’t sell them. But they decide which ones hospitals and pharmacies can stock.
They push for the cheapest options. Always. Even if a drug has a history of shortages. Even if another version is more reliable. They get paid based on volume and price, not quality or safety. So they choose the drug that costs the least today-even if it’s the one that keeps disappearing.
And they don’t tell anyone why. Hospitals don’t know why certain drugs are excluded from formularies. Pharmacists can’t explain why a patient’s usual medication suddenly isn’t covered. The system is opaque. And it’s designed to cut costs, not prevent crises.
The Federal Trade Commission called this out in 2023, saying PBMs make ‘critical decisions about access to life-saving medications without transparency or accountability.’ That’s not just bad policy. It’s dangerous.
The Result? Patients Pay the Price
When a drug disappears, it’s not just a logistics problem. It’s a medical emergency.
Hospital pharmacists now spend up to 75% more time managing shortages than they did 10 years ago. That’s time they’re not spending counseling patients, checking for interactions, or ensuring safety. Instead, they’re calling other hospitals, begging for extra stock, switching patients to alternatives with different side effects, or rationing doses.
One study found that one in four shortage reports had no explanation at all. No cause. No timeline. No plan. Just silence. Patients with cancer, heart disease, or chronic infections are left in limbo. Doctors are forced to choose between delayed treatment and risky substitutions.
And it’s not just the U.S. Canada has similar shortages-but handles them better. Why? Because they have a national stockpile. Because regulators, hospitals, and manufacturers talk to each other. Because they plan ahead. The U.S. doesn’t. Its stockpile is only for bioterrorism or natural disasters-not for a missing antibiotic.
What’s Being Done? Not Enough
There are proposals. The RAPID Reserve Act, introduced in 2023, wants to create a federal stockpile of critical generic drugs. It wants to offer tax breaks to companies that make drugs domestically. It wants to require manufacturers to report potential shortages six months in advance.
But none of it’s law yet. And even if it passes, it won’t fix the root problem: the economics of generic drugs.
As long as manufacturers are forced to sell life-saving medicines for pennies, they’ll keep cutting corners. As long as PBMs choose price over reliability, shortages will keep happening. As long as 80% of active ingredients come from two countries with no backup, the system will remain fragile.
Real change means paying more for generic drugs. Not a lot more. Just enough to cover the cost of safe, reliable production. Enough to keep factories open. Enough to hire trained staff. Enough to build redundancy. Enough to make sure the next time a machine breaks, there’s another one ready to go.
It’s not about making drug companies rich. It’s about making sure patients don’t die because no one was willing to pay a fair price for a pill.





Julia Jakob
December 4, 2025 AT 06:38David Ross
December 4, 2025 AT 11:05Sophia Lyateva
December 6, 2025 AT 09:33Rachel Nimmons
December 6, 2025 AT 17:12Abhi Yadav
December 8, 2025 AT 01:52Krys Freeman
December 8, 2025 AT 04:24Jerry Ray
December 9, 2025 AT 19:10gladys morante
December 11, 2025 AT 00:44Melania Dellavega
December 12, 2025 AT 02:54Bethany Hosier
December 12, 2025 AT 11:12Shawna B
December 13, 2025 AT 16:33Lyn James
December 15, 2025 AT 03:57Craig Ballantyne
December 15, 2025 AT 21:01Victor T. Johnson
December 17, 2025 AT 19:26Nicholas Swiontek
December 18, 2025 AT 21:21