Every year, Americans fill over 4 billion prescriptions for generic drugs. These are the same medicines as brand-name pills, but cost up to 85% less. Behind the scenes, a quiet but powerful system keeps these drugs flowing: the Generic Drug User Fee Amendments, or GDUFA. Without it, many of these life-saving medications would still be sitting on a shelf, waiting years for approval.
Why the FDA Needs Money to Approve Generic Drugs
Before 2012, the FDA’s generic drug office was underfunded and overwhelmed. Applications piled up. Some took over three years just to get reviewed. Companies didn’t know what the FDA wanted. Delays meant patients waited longer for cheaper drugs. The system wasn’t broken-it was starved. Congress fixed that by letting the FDA collect fees directly from generic drug makers. This isn’t a tax. It’s a user fee. If you want the FDA to review your drug application, you pay for the service. That money goes straight into the Office of Generic Drugs. Today, about 75% of its budget comes from these fees. The rest is from Congress. This model gives the FDA predictable funding so it can hire reviewers, upgrade systems, and meet deadlines.How GDUFA Works: The Four Fees
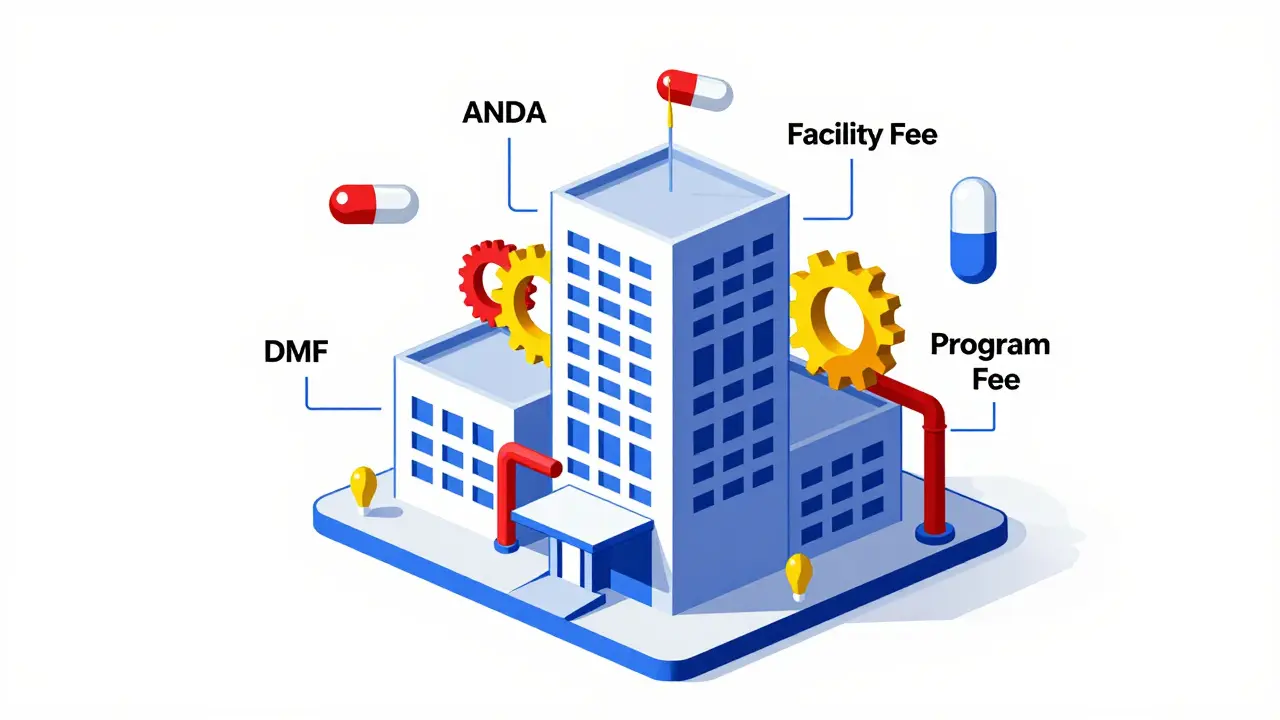
GDUFA doesn’t charge one flat fee. It has four types, each tied to a different part of the process:- Application fee: $124,680 per Abbreviated New Drug Application (ANDA). This is what you pay when you submit your generic drug for approval. It covers the cost of reviewing your data, chemistry, and manufacturing.
- Program fee: $385,400 per year for any company with an approved generic drug. This pays for the overall program-staff, training, oversight.
- Facility fee: $25,850 per manufacturing site that makes the active ingredient or finished pill. If your drug is made in a factory, that factory pays this fee. Even if it’s not your own facility, if it’s listed in your application, you’re responsible.
- DMF fee: $25,850 for each Drug Master File (DMF) that’s referenced. These are technical dossiers on ingredients like active pharmaceutical ingredients (API). You pay when you first use a DMF in your application.
These fees are adjusted every year based on inflation. The FDA publishes exact numbers in its annual guidance. Payments are made through an online system called EUF. Miss a deadline? Your application won’t be reviewed until you pay.
Speeding Up Approval: The 15-Month Goal
One of GDUFA’s biggest wins is time. Before 2012, the average review took 30 to 36 months. Now, the goal is to finish 60% of original ANDA applications within 15 months. The FDA tracks this closely. In 2021, they hit 52%-close, but not quite there. Pandemic delays and more complex applications pushed it back.But even that 52% is a huge leap. In 2022, the FDA received 1,128 generic drug applications. That’s over 16 times more than the number of brand-name drug applications. Without GDUFA, that volume would have crushed the system. Now, companies get clearer feedback. Deficiency letters-those lists of changes the FDA wants-are more specific. One Teva manager said in 2023 that 90% of these letters now include actionable steps, not vague comments.

Who Pays? And Who Gets Left Behind?
Big companies like Teva, Sandoz, and Mylan can handle these fees. They have hundreds of approved drugs. But small manufacturers? It’s harder.Imagine a small company with one facility and only two approved generics. Their facility fee alone is $25,850. Their program fee is another $385,400. That’s over $400,000 just to stay in the game. For a small firm, that’s 15% of their entire regulatory budget. Some have to delay expansions or skip new applications.
The FDA knows this. There’s a 75% fee reduction for small businesses that meet certain criteria-fewer than 500 employees, no more than three approved drugs. But in 2022, only 18 small companies used it. Why? Many don’t know it exists. Others think the paperwork is too messy. The FDA offers webinars and help desks, but the learning curve is still 3 to 6 months for new staff.
What GDUFA Doesn’t Cover
GDUFA only applies to prescription generics. It doesn’t touch over-the-counter (OTC) drugs-things like pain relievers, antacids, or allergy meds sold without a prescription. That’s a $117 billion market. These drugs follow older, slower rules called monographs. There’s no user fee system. No deadlines. No funding boost. Experts say bringing OTC drugs under GDUFA could generate $150-200 million a year and fix a broken system.Right now, if you want to make a new generic antacid, you’re stuck in a bureaucratic maze. No one’s paying for faster review. Patients wait. Companies can’t innovate. It’s a blind spot in an otherwise strong program.
The Real Impact: More Drugs, Lower Prices
The numbers tell the real story. Since GDUFA started, generic drug approvals have jumped 22% annually. The FTC says GDUFA helped speed up generic entry after brand-name patents expired-by 15%. That’s saved consumers $1.7 trillion in the last decade.Generic drugs make up 90% of all prescriptions in the U.S. But they cost only 23% of the total drug spending. That’s because GDUFA keeps the pipeline full. When a drug goes generic, prices drop fast. Sometimes by 90% in the first year. That’s not luck. That’s policy.
And it’s working. The Congressional Budget Office says GDUFA pays for itself. For every dollar the FDA spends on generic reviews, $1.20 comes in from user fees. That’s a win for taxpayers. It’s also a win for patients who need affordable medicines.
What’s Next? GDUFA III and Beyond
GDUFA was renewed in 2022 and runs through 2027. This version, called GDUFA III, added new tools:- DMF completeness assessments-now you can get feedback on your ingredient file before submitting your full drug application.
- Clearer inspection schedules: finished drug sites get checked every two years, API sites every three.
- A plan to eliminate all pre-2012 backlog applications by September 2024.
Future talks are already starting for GDUFA IV. One idea: using real-world data from pharmacies and electronic health records to monitor generic drug safety after they’re on the market. But industry groups warn that could add cost and complexity. The FDA is listening.
One thing’s clear: GDUFA isn’t perfect. But it’s the best system we have. It turned a broken, slow process into a predictable, faster one. It didn’t just help companies-it helped patients. And as long as the fees keep flowing, more affordable drugs will keep reaching shelves.





Mike Rengifo
December 18, 2025 AT 23:43Kinda wild how something so dry like user fees ends up saving people thousands on their prescriptions. I never thought about how much paperwork goes into a $3 pill.
Meenakshi Jaiswal
December 19, 2025 AT 23:32This is actually one of the most effective public health policies we’ve got. The FDA doesn’t get rich off this - they just get the resources to do their job. Small pharma needs more outreach, but the framework? Solid.
jessica .
December 21, 2025 AT 16:14so the fda is basically a toll booth for big pharma and we call it 'public service'? 🤡
Andrew Kelly
December 23, 2025 AT 14:06Of course the conspiracy theorists are out in full force. Let me guess - next you’ll say the FDA is secretly controlled by Big Pharma to keep generics expensive? Newsflash: the fees are public, the timelines are published, and the savings are quantifiable. You’re mad because the system works too well for you to hate it.
mary lizardo
December 24, 2025 AT 02:31The entire premise of GDUFA is a grotesque commodification of public health. To charge pharmaceutical entities for the mere act of reviewing life-saving therapeutics is to institutionalize the notion that access to medicine is contingent upon financial capacity - a moral abdication disguised as fiscal pragmatism. One cannot, in good conscience, equate regulatory diligence with a service transaction.
Moreover, the so-called 'efficiency gains' are statistically inflated by the exclusion of OTC drugs, which remain mired in archaic monograph systems. This selective reform is not progress - it is performative governance.
The FDA, as a public institution, ought to be funded by the public purse, not by the very corporations it is meant to regulate. The conflict of interest is not merely theoretical; it is structural.
And yet, we are told to celebrate the 15-month review cycle as a triumph. A triumph of bureaucracy over benevolence. A triumph of metrics over morality.
When the cost of approval exceeds the profit margin of a small manufacturer, we do not have a functioning system - we have a gatekeeping mechanism disguised as a regulatory framework.
Let us not confuse speed with justice. Let us not mistake transparency for equity. And let us not, under any circumstances, mistake user fees for public good.
It is not a win when patients receive cheaper drugs because the system was bribed into efficiency. It is a tragedy that it took bribes to get there at all.
The real scandal isn’t the delay - it’s that we’ve normalized paying for the right to live.
Isabel Rábago
December 25, 2025 AT 11:07People act like GDUFA is some miracle, but it’s just the government doing what it should’ve done in the first place - fund its own agencies. If we spent half as much on public health infrastructure as we do on military contractors, we wouldn’t need user fees to begin with.
And don’t get me started on how 'small business' is defined. 500 employees? In pharma, that’s a startup with three people in a garage. The real small players - the ones with 20 employees and one generic - are still getting crushed.
Also, why is no one talking about how the FDA’s inspection backlog is still growing? They review faster, but they’re still falling behind on factories. That’s a ticking time bomb.
And yes, I know I’m the only one who remembers the 2008 heparin crisis. That’s what happens when you outsource manufacturing to countries with zero oversight. GDUFA didn’t fix that. It just made the paperwork prettier.
Anna Sedervay
December 27, 2025 AT 00:07One must question the epistemological foundations of GDUFA’s efficacy metrics. The 15-month benchmark, while statistically impressive, is predicated upon a reductionist operationalism that ignores the qualitative dimensions of drug safety - namely, the subtle pharmacokinetic variances that may emerge post-marketing. Furthermore, the omission of OTC drugs from this regulatory architecture constitutes a profound structural lacuna, one that perpetuates a bifurcated pharmacopeia wherein prescription generics are privileged over non-prescription therapeutics - a hierarchy that is neither scientifically nor ethically defensible.
It is, therefore, not merely a fiscal arrangement, but a hegemonic reconfiguration of pharmaceutical governance - one wherein market logic supplants public health imperatives.
Matt Davies
December 28, 2025 AT 06:31Man, this is the kind of policy that makes me proud to be from a country that still tries to make healthcare work for people, not just profits. The fact that we’ve saved $1.7 trillion in a decade because someone had the brains to fund the FDA properly? That’s not just smart - it’s beautiful.
And yeah, small companies are getting squeezed. But that’s not GDUFA’s fault - it’s the lack of outreach. Fix the help desks, not the system. This thing is basically the superhero origin story of affordable medicine.
Ashley Bliss
December 28, 2025 AT 13:19They say GDUFA saved lives. But who saved GDUFA? Who fought for it? Who sat in those congressional hearings while lobbyists whispered sweet nothings into senators’ ears? Someone had to. Someone always does.
And now? We’re told to clap because the machine works. But machines don’t care who gets left behind. They just run.
I wonder if the man who invented the first generic pill ever imagined his life’s work would be priced by a fee schedule.
Maybe we should’ve just made it free.
Maybe we should’ve just said: medicine is a right, not a transaction.
But no. We made it a business. And now we’re surprised when the business eats the patient.
And still… we call it progress.
What a world.
Dev Sawner
December 28, 2025 AT 18:48Statistical analysis of GDUFA’s cost-benefit ratio reveals an implicit subsidy to multinational pharmaceutical corporations through regulatory capture. The fee structure disproportionately benefits firms with economies of scale, while the small business exemption is rendered functionally inert due to administrative opacity. The 75% reduction rate, though nominally generous, is inaccessible to 92% of eligible entities due to compliance burden. This constitutes a regressive policy disguised as reform.
Furthermore, the absence of OTC integration represents a critical failure in holistic pharmaceutical governance. The monograph system, established in 1972, remains unamended despite technological advances - a regulatory anachronism.
Conclusion: GDUFA is a superficial optimization that entrenches structural inequities under the guise of efficiency.
Moses Odumbe
December 29, 2025 AT 15:29bro the FDA is basically a subscription service now 😂💸
but honestly… if I pay $3 for my blood pressure med instead of $300, i’m not complaining. 🙌
bhushan telavane
December 29, 2025 AT 20:47India makes over 60% of the world’s generic drugs. We know how hard it is to meet FDA standards. These fees? They’re tough, but they force quality. No one wants a bad batch of metformin.
Just wish the FDA would do more to help small Indian manufacturers understand the system. A lot of us don’t even know where to start.
Mahammad Muradov
December 30, 2025 AT 20:17The entire GDUFA framework is a facade of efficiency. The FDA’s increased throughput is achieved by prioritizing applications from corporations with legal departments, while rejecting or delaying submissions from entities lacking in-house regulatory affairs teams. The 15-month timeline is a marketing metric - not a guarantee of safety or equivalence. The system incentivizes form over function, compliance over innovation. This is not progress. It is corporate capture dressed in bureaucratic clothing.
Isabel Rábago
January 1, 2026 AT 01:18And now they’re talking about using real-world data from pharmacies to monitor safety after approval? That’s brilliant - if they actually fund it. Otherwise it’s just another promise on a PowerPoint slide.