Key Takeaways
- Everolimus is a newer, more water‑soluble analogue of sirolimus, offering tighter dosing control.
- Both drugs inhibit the mTOR pathway, but their Approved indications differ: sirolimus is classic for kidney transplants, everolimus shines in oncology and certain organ transplants.
- Pharmacokinetics vary: everolimus has a shorter half‑life (~30 h) versus sirolimus (~60 h), influencing monitoring frequency.
- Adverse‑effect profiles overlap (mouth ulcers, hyperlipidaemia) yet everolimus tends to cause more metabolic disturbances, while sirolimus is linked to delayed wound healing.
- Choosing the right agent hinges on the clinical scenario, renal function, and drug-drug interaction landscape.
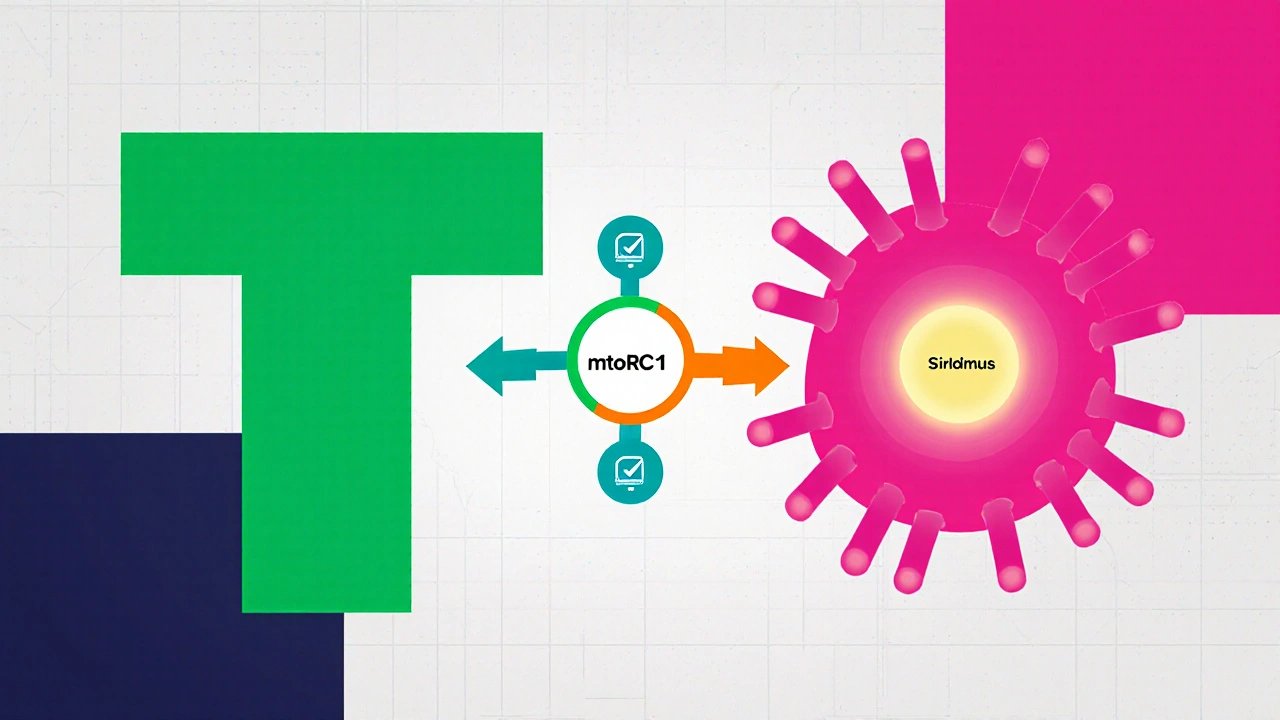
When doctors need to suppress the immune system or slow tumor growth, Everolimus is a derivative of sirolimus that acts as an mTOR inhibitor, used primarily in oncology and transplant medicine often pops up alongside its older sibling, Sirolimus is a natural macrolide discovered in the 1970s, also known as rapamycin, that blocks the same mTOR pathway but with a different chemical backbone. Understanding how they differ can feel like untangling a knot of chemistry, dosing charts, and FDA approvals. This guide walks through the science, the real‑world uses, and the practical pros‑cons so you can see why a physician might pick one over the other.
How Both Drugs Hit the mTOR Pathway
The mammalian target of rapamycin (mTOR) is a cellular hub that tells cells when to grow, divide, or rest. When a transplant organ arrives, the immune system’s T‑cells sense the foreign tissue and fire up mTOR, leading to rejection. Cancer cells also hijack mTOR to fuel rapid growth. Both everolimus and sirolimus bind to the intracellular protein FKBP12; this complex then latches onto mTOR complex 1 (mTORC1) and shuts it down.
The big difference is chemistry. Sirolimus is a large, lipophilic molecule that sticks around in fatty tissues, while everolimus has a 2‑hydroxyethyl side chain that makes it more water‑soluble. That tweak improves oral absorption and lets doctors fine‑tune blood levels more precisely.
Approved Clinical Uses: Where Each Shines
Everolimus vs sirolimus isn’t just a lab talk - the FDA has carved distinct niches for them. Sirolimus earned its first approval in 1999 for kidney‑transplant prophylaxis. It’s still a go‑to for preventing rejection in liver, heart, and pancreas transplants, especially when combined with calcineurin inhibitors.
Everolimus entered the market later, in 2009, with a two‑pronged strategy: 1) as an oncology drug for advanced renal cell carcinoma, and 2) as an adjunct immunosuppressant for kidney‑transplant patients who need less nephrotoxicity. Since then, it’s added approvals for breast cancer, pancreatic neuroendocrine tumors, and subependymal giant cell astrocytoma associated with tuberous sclerosis.
In practice, a transplant surgeon might stick with sirolimus for a standard kidney graft, while an oncologist will likely reach for everolimus when a patient’s tumor shows mTOR pathway activation.
Pharmacokinetics and Dosing Regimens
All that chemistry shows up in how the drugs behave in the body. Sirolimus has a long elimination half‑life of roughly 60 hours, meaning steady‑state can take up to two weeks. Blood levels are usually kept between 5‑15 ng/mL for transplant patients, with weekly monitoring.
Everolimus clears faster, with a half‑life around 30 hours. The therapeutic window is tighter - 3‑8 ng/mL for most transplant protocols, and 5‑15 ng/mL for cancer indications. Because of the shorter half‑life, dose adjustments can be made more quickly, and some clinicians appreciate the reduced risk of over‑suppression.
Both drugs are taken orally, but food effects differ. Sirolimus absorption declines by about 30 % with a high‑fat meal, so the recommendation is to take it on an empty stomach. Everolimus is less sensitive to food, yet still benefits from consistent timing each day.
Safety Profiles: Overlapping Risks and Unique Concerns
Because the mechanisms converge, many side‑effects overlap. Common adverse events include mouth ulcers, acne‑like rash, hyperlipidaemia, and peripheral edema. Both can impair wound healing - a critical point for post‑surgical transplant patients.Everolimus tends to cause more metabolic disturbances: higher rates of hyperglycaemia, new‑onset diabetes, and increased serum triglycerides. Sirolimus, on the other hand, shows a stronger link to delayed wound healing and lymphocele formation, especially after kidney transplant.
Drug-drug interactions are another arena where they differ. Both are metabolized by CYP3A4, so strong inhibitors (ketoconazole, clarithromycin) raise levels, while inducers (rifampin, carbamazepine) lower them. Everolimus, being more water‑soluble, is also a substrate for the drug transporter P‑gp; inhibitors like verapamil can push blood concentrations up dramatically.
Side‑by‑Side Comparison Table
| Attribute | Everolimus | Sirolimus |
|---|---|---|
| Chemical class | Rapalog (2‑hydroxyethyl derivative) | Macrolide (natural rapamycin) |
| Half‑life | ≈30 hours | ≈60 hours |
| Key FDA approvals (US) | Renal cell carcinoma, breast cancer, transplant adjunct, tuberous sclerosis‑related brain tumors | Kidney, liver, heart, pancreas transplant prophylaxis |
| Typical transplant dose | 0.75 mg twice daily (adjust to 3‑8 ng/mL) | 2 mg once daily (adjust to 5‑15 ng/mL) |
| Common adverse effects | Mouth ulcers, hyperglycaemia, hyperlipidaemia, infections | Mouth ulcers, delayed wound healing, lymphocele, hyperlipidaemia |
| Major drug interactions | CYP3A4 & P‑gp inhibitors (e.g., verapamil) increase levels | CYP3A4 inhibitors (e.g., ketoconazole) increase levels |
Practical Decision‑Making: Which One Fits Your Situation?
When a clinician weighs options, a few practical questions surface:
- What is the primary goal? For pure immunosuppression in a stable kidney transplant, sirolimus remains the classic choice. If the patient also has a malignancy sensitive to mTOR inhibition, everolimus can kill two birds with one stone.
- How robust is the patient’s hepatic function? Both drugs rely on CYP3A4, but everolimus’s shorter half‑life makes dose tweaking easier in liver impairment.
- Is wound healing a concern? Post‑operative patients may benefit from sirolimus avoidance for the first 6 weeks, whereas everolimus can be introduced sooner due to its milder impact on fibroblast activity.
- Are there metabolic risks? For patients with pre‑existing diabetes or high triglycerides, sirolimus might be a safer bet, provided the surgeon is comfortable with its wound‑healing profile.
- What other meds are in the mix? A comprehensive review of CYP3A4 and P‑gp modulators can tip the scales; everolimus is more sensitive to P‑gp inhibition, so strong P‑gp inhibitors may necessitate dose cuts.
Bottom line: there’s no one‑size‑fits‑all answer. The choice is a balancing act between efficacy, safety, and the patient’s comorbidities.
Frequently Asked Questions
Can I switch from sirolimus to everolimus?
Yes, many physicians transition patients when a cancer indication emerges or when they need tighter therapeutic monitoring. The switch requires a wash‑out period of about 48 hours, followed by re‑checking trough levels to avoid over‑immunosuppression.
Do both drugs cause the same infection risk?
Both increase susceptibility to opportunistic infections such as CMV or fungal infections, but the magnitude is comparable. Prophylactic antivirals are standard for both, especially in the first six months post‑transplant.
Which drug is more expensive?
Pricing varies by country and insurance coverage, but generally everolimus is priced slightly higher due to its newer status and oncology indications. Generic sirolimus has been available for longer, often lowering its cost.
Is therapeutic drug monitoring required for both?
Absolutely. Both have narrow therapeutic windows, and blood level checks guide dosing. Everolimus often needs more frequent monitoring early on because of its faster kinetics.
Can pregnant women take either medication?
Both are classified as Pregnancy Category C; they cross the placenta and have shown teratogenic effects in animal studies. They should be avoided unless the benefit clearly outweighs the risk, and only under specialist supervision.
Understanding the nuances between everolimus and sirolimus equips clinicians, patients, and caregivers to make smarter, safer choices. Whether you’re navigating a transplant protocol or an oncology regimen, the right drug can mean better outcomes and fewer surprises.

Carla Taylor
October 24, 2025 AT 16:52Great summary of the everolimus and sirolimus landscape it really helps to see the big picture at a glance. The way you laid out the pharmacokinetic differences makes it easy to remember which drug fits which scenario. I appreciate the clear headings and the practical tips on dosing timing.
Mary Mundane
November 3, 2025 AT 17:32Honestly the article could have cut the fluff.
Jacqueline Galvan
November 13, 2025 AT 19:12Allow me to expand on several points that are crucial for clinicians choosing between everolimus and sirolimus. First, the structural modification that introduces a hydroxyethyl side chain to everolimus not only improves aqueous solubility but also reduces its affinity for adipose tissue, thereby shortening the terminal elimination phase. In contrast, sirolimus’s highly lipophilic macrocyclic lactone backbone leads to extensive tissue sequestration, which accounts for its protracted half‑life of approximately 60 hours. This pharmacokinetic disparity translates directly into monitoring frequency; everolimus levels can be adjusted on a weekly basis, whereas sirolimus often requires a bi‑weekly or even monthly assessment to achieve steady‑state. Second, the therapeutic windows differ markedly. Everolimus targets a narrower concentration range (3‑8 ng/mL for transplant protocols) compared with sirolimus’s broader range (5‑15 ng/mL), demanding more precise dose titration but also offering the advantage of quicker correction in the event of toxicity. Third, the side‑effect profiles, while overlapping, have nuanced distinctions. Everolimus is associated with a higher incidence of dyslipidemia and hyperglycemia, likely due to its impact on the AKT‑mTOR axis in metabolic tissues, whereas sirolimus more frequently impairs wound healing, a consideration of paramount importance in the immediate postoperative period for transplant recipients. Fourth, regulatory approvals guide clinical use: sirolimus remains the cornerstone for prophylaxis in kidney, liver, heart, and pancreas transplants, especially when used in combination with calcineurin inhibitors. Meanwhile, everolimus has carved out a niche in oncology, receiving FDA clearance for advanced renal cell carcinoma, hormone‑receptor positive breast cancer, and subependymal giant cell astrocytoma in tuberous sclerosis. Fifth, drug‑drug interactions must be meticulously reviewed. Both agents are metabolized by CYP3A4 and are substrates for P‑gp; however, everolimus’s shorter half‑life makes it somewhat more forgiving when co‑administered with strong CYP3A4 inhibitors or inducers. Finally, patient-specific factors such as baseline renal function, lipid profile, and surgical timeline should drive the ultimate decision. In patients with marginal renal reserve, everolimus may offer a nephroprotective advantage, while those undergoing major surgery may benefit from the more gradual immunosuppression of sirolimus. In summary, the choice between everolimus and sirolimus is not merely a matter of brand preference but a complex interplay of pharmacology, clinical indication, and individual patient characteristics.
Dawn Bengel
November 23, 2025 AT 20:52All this talk about “choose wisely” is just academic – you’ll see the American market dominate with everolimus and ignore the proven track record of sirolimus. 🇺🇸💪
junior garcia
December 3, 2025 AT 22:32Wow, this guide is like a roller‑coaster of science! I can feel the drama in every half‑life number – it’s almost poetic how the drugs dance inside our bodies. Seriously, the way you explained the water‑solubility tweak was pure brilliance.
Casey Morris
December 14, 2025 AT 00:12Indeed, the nuanced differences you present are essential; however, one must also consider the socioeconomic factors influencing drug accessibility, which often dictate real‑world usage patterns; thus, the academic discourse must be tempered with pragmatic considerations.
Teya Arisa
December 24, 2025 AT 01:52Thank you for this thorough overview; it provides clinicians with a clear framework to evaluate both agents. Your emphasis on monitoring frequencies and side‑effect nuances will certainly aid in optimizing patient outcomes. 😊
Kester Strahan
January 3, 2026 AT 03:32Yo, the mTOR inhibittion rift is real – everolimus is like the fast‑track ticket while sirolimus is the OG slow‑burn. Gotta keep an eye on those CYP3A4 interactions, otherwise u’ll be in a s**tstorm.
Doreen Collins
January 13, 2026 AT 05:12From a coaching perspective, the decision tree you outlined serves as an excellent playbook. Imagine guiding a new transplant fellow through these criteria; the clarity you provide accelerates confidence and competence.
HILDA GONZALEZ SARAVIA
January 23, 2026 AT 06:52Expanding on the playbook idea, consider integrating a decision matrix that scores each drug on parameters such as renal impact, metabolic side effects, and wound‑healing risk. This quantifiable approach can transform subjective judgment into objective guidance, especially for multidisciplinary teams.
Amanda Vallery
February 2, 2026 AT 08:32Ths artcle msisst a ptic al lnk btwn cpture and dsease.
Marilyn Pientka
February 12, 2026 AT 10:12It is morally indefensible to overlook the ethical implications of prescribing immunosuppressants without rigorous patient education. The industry’s profit‑driven narrative must be challenged, and clinicians bear the responsibility to uphold a higher standard of informed consent, lest we compromise the very foundations of medical ethics.
Jordan Levine
February 22, 2026 AT 11:52THIS IS THE REAL DEAL! EVEROLIMUS IS THE FUTURE AND SIROLIMUS IS JUST A LEGACY LEFTOVER! 💥💥💥