When your hand starts shaking without you meaning it to - especially when you’re sitting still - it’s easy to brush it off as nerves or fatigue. But if that tremor sticks around, gets worse, and is joined by stiffness, slow movements, or trouble balancing, it might not be just aging. For more than 10 million people worldwide, this is Parkinson’s disease. It doesn’t just shake your hand. It shakes your routine, your independence, your sense of control.
What’s Really Happening in the Brain?
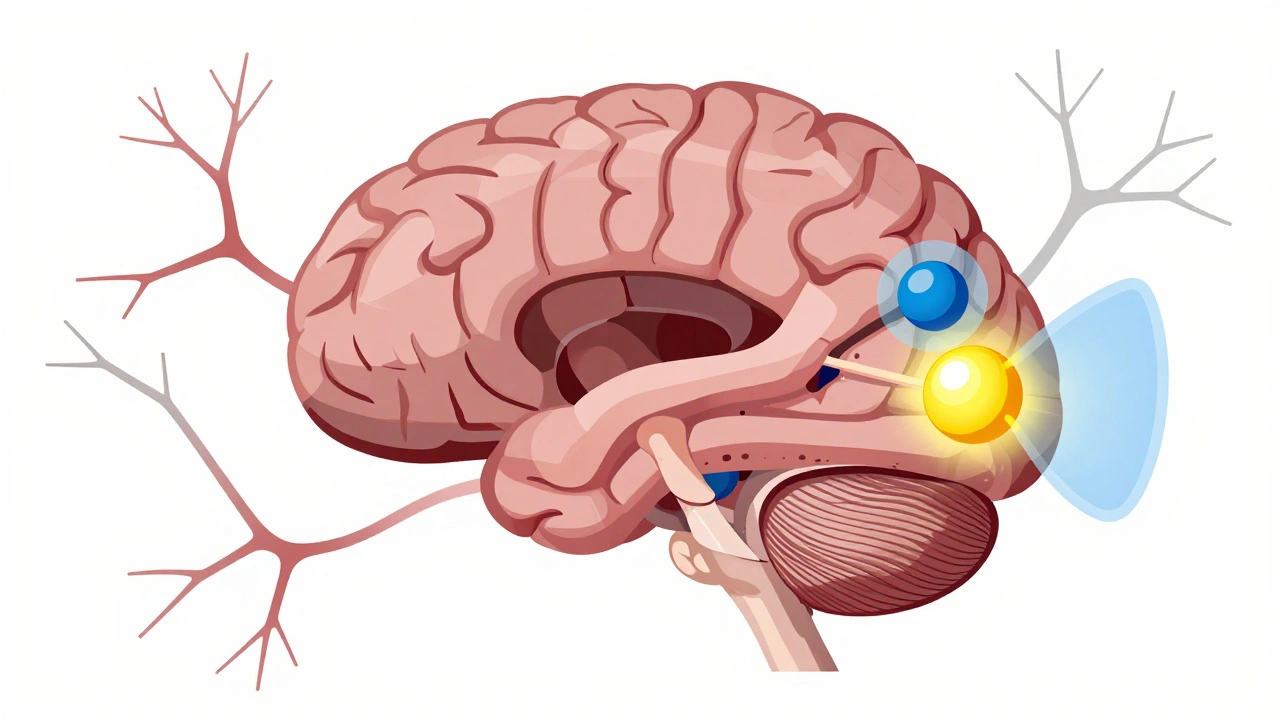
Parkinson’s isn’t a mystery anymore. We know it’s about a slow, steady loss of nerve cells in a tiny part of the brain called the substantia nigra. These cells make dopamine - a chemical messenger that tells your muscles when and how to move. By the time someone starts showing symptoms like tremor or stiffness, they’ve already lost 60 to 80% of those dopamine-producing cells. That’s not a small drop. It’s a collapse in the system that keeps your body moving smoothly.Without enough dopamine, the brain’s movement circuits get out of sync. The signals get mixed up. Your muscles don’t get the clear go-ahead to move, so they freeze up. Or they overreact, shaking on their own. This isn’t just weakness. It’s a wiring problem.
The Three Big Signs: Tremor, Stiffness, and Slowness
There are four main motor symptoms, but three stand out early on: tremor, rigidity, and bradykinesia.Tremor is what most people picture - the hand shaking like you’re holding a cup of coffee you’re too nervous to drink. But it’s not just any shake. It’s a resting tremor, meaning it happens when your hand is relaxed, not when you’re using it. It often starts in just one hand, usually with a pill-rolling motion: thumb and forefinger rubbing together like you’re trying to crush a tiny pill. It fades when you reach for something, and it disappears while you sleep. Stress, fatigue, or strong emotions can make it worse. About 80% of people with Parkinson’s get this kind of tremor.
Stiffness - or rigidity - is harder to ignore. It’s not just tight muscles. It’s your arms or legs feeling like they’re stuck in concrete. When a doctor moves your limb during an exam, it might feel like a cogwheel - a jerky, stop-and-go resistance - or like pushing through lead pipe stiffness. This isn’t just uncomfortable. It makes everyday things impossible: buttoning a shirt, turning a key, writing your signature. A study from Parkinson’s UK found that 73% of people struggle with these small tasks within three years of diagnosis.
Slowness of movement (bradykinesia) is the silent thief. You don’t realize how much you’ve slowed down until you try to get out of a chair or walk across the room. Steps become small and shuffling. Your face loses expression. Your voice gets quieter. It’s not laziness. It’s your brain struggling to start and sustain movement.
Dopamine Replacement: The Main Tool We Have
Since we can’t bring back dead brain cells, the goal is simple: replace what’s missing. That’s where dopamine replacement therapy comes in.The gold standard is levodopa - a chemical your body turns into dopamine. But levodopa alone won’t work. If you take it by itself, your body converts it into dopamine in your blood before it even reaches your brain. That causes nausea, low blood pressure, and other side effects. So it’s always paired with carbidopa, which blocks that early conversion. Together, they’re sold as Sinemet, Rytary, or generic versions. The typical ratio is 4 parts levodopa to 1 part carbidopa.
For most people, levodopa works like magic - at first. Within 30 to 60 minutes, stiffness eases, tremor slows, and movement feels possible again. Studies show up to 70% improvement in motor symptoms during the early years. This is called the “honeymoon period.” It can last 5 to 10 years. For many, it’s the difference between living at home and needing help.
The Catch: What Happens When the Magic Fades?
But levodopa doesn’t stop the disease. It just masks the symptoms. And over time, the brain gets less able to handle it.After several years, two big problems show up:
- Wearing-off: The medicine doesn’t last as long. You might feel fine for two hours, then suddenly freeze up before your next dose. This isn’t just inconvenient - it’s terrifying. You can’t predict when it’ll happen.
- Dyskinesias: Your body starts moving on its own. Twitches, jerks, waves - involuntary movements that can be as disruptive as the original symptoms. They usually happen when levodopa levels are highest.
By the 10-year mark, 40 to 50% of people on levodopa experience these complications. And they’re not rare. Reddit users in r/Parkinsons talk about it every day. One person wrote: “After 8 years, my ‘on’ time dropped from 6 hours to 2 or 3. Now I’m shaking uncontrollably at peak dose.”
Alternatives to Levodopa
Not everyone starts with levodopa. Especially younger patients. That’s because the longer you wait, the longer you can delay those motor complications.Dopamine agonists like pramipexole and ropinirole trick your brain’s dopamine receptors into thinking dopamine is there. They’re not as strong as levodopa - about 30 to 50% as effective - but they come with a lower risk of early dyskinesias. Many people start with these to buy time. But they have their own side effects: dizziness, sleepiness, hallucinations, and even impulse control issues like compulsive gambling or shopping.
Some people end up needing both. A 2023 report from the Cleveland Clinic says 60% of patients eventually use levodopa and a dopamine agonist together. It’s not ideal, but it’s often necessary.

How to Take It Right - And Avoid Common Mistakes
Taking levodopa isn’t just popping a pill. Timing matters more than you think.High-protein meals - steak, eggs, cheese, beans - compete with levodopa for absorption in the gut. If you take your pill with a burger, it might not work. Many people learn the hard way: take levodopa 30 to 60 minutes before eating, or at least 1 hour after. It’s a small change, but it can mean the difference between a good day and a frozen one.
Dosing is another challenge. The American Parkinson Disease Association recommends starting low: 25/100 mg once or twice a day. Then slowly increase. Many doctors still start too high, rushing to fix symptoms. But a 2023 survey found 85% of movement disorder specialists now use a “start low, go slow” approach. It reduces side effects and delays complications.
And then there’s the cost. Generic levodopa/carbidopa costs about $600 a year. But extended-release versions like Rytary? Around $5,800. Inbrija, the inhaled levodopa for sudden “off” episodes, runs $3,700 a month. Insurance doesn’t always cover it. Many patients spend hours managing schedules, calling pharmacies, or asking caregivers to remind them. The Parkinson’s Foundation says 78% need help managing meds.
What’s Next? The Future of Treatment
We’re not stuck with pills. New options are emerging.In 2018, the FDA approved Inbrija - a powder you inhale when you suddenly freeze up. It kicks in within 10 minutes. It’s not a replacement for daily pills, but it’s a lifeline for those unpredictable “off” moments.
More exciting is subcutaneous infusion. The 2022 RESTORE-1 trial tested a pump that delivers a continuous dose of levodopa under the skin. Results showed patients gained 2.5 extra “on” hours per day. No more waiting for the next pill. No more sudden freezes. It’s not widely available yet, but it’s a glimpse of what’s coming.
Gene therapies are also being tested - trying to get the brain to make its own dopamine again. And researchers are using genetic testing to predict who will respond best to which drug. If you have a certain variant in your COMT or MAO-B genes, you might do better on one medication than another. Personalized treatment is no longer science fiction.
Living With It
Parkinson’s isn’t a death sentence. But it’s a lifelong adjustment. The goal isn’t to cure it - not yet. It’s to keep you moving, independent, and in control for as long as possible.Levodopa still works. It’s not perfect. It’s not forever. But for now, it’s the best tool we have. The key isn’t just taking it - it’s taking it right. Timing. Dosing. Diet. Monitoring. And knowing when to add another layer of treatment.
Every person’s journey is different. One person manages well for 10 years on pramipexole. Another needs levodopa within a year. There’s no one-size-fits-all. That’s why working with a specialist - a neurologist who focuses on movement disorders - makes all the difference.
The disease will change. Your treatment will change. But you don’t have to lose control. With the right plan, you can still walk, talk, and live - even as the tremor lingers.
Is Parkinson’s disease curable?
No, Parkinson’s disease is not currently curable. It’s a progressive condition caused by the loss of dopamine-producing nerve cells in the brain. While treatments like levodopa can effectively manage symptoms and improve quality of life, they do not stop or reverse the underlying nerve damage. Research into gene therapy, stem cells, and neuroprotective drugs is ongoing, but no cure exists as of 2025.
Why does levodopa stop working over time?
Levodopa doesn’t stop working because the body rejects it - it’s because the brain changes. As more dopamine cells die, the brain loses its ability to store and release dopamine smoothly. This leads to “wearing-off,” where the drug’s effect doesn’t last as long, and “on-off” fluctuations, where symptoms suddenly return. Over time, the brain also becomes more sensitive to high dopamine levels, causing involuntary movements called dyskinesias. These aren’t signs the drug failed - they’re signs the disease progressed.
Can diet affect Parkinson’s medication?
Yes, protein can interfere with levodopa absorption. Amino acids from meat, dairy, and legumes compete with levodopa for the same transporters in the gut and blood-brain barrier. Taking levodopa 30 to 60 minutes before meals or 1 hour after eating helps avoid this. Some people follow a protein-redistribution diet - eating most protein at dinner - to improve daytime symptom control. Always discuss dietary changes with your doctor or a dietitian familiar with Parkinson’s.
Are dopamine agonists better than levodopa?
Not necessarily - they’re different tools. Dopamine agonists like pramipexole are less effective at controlling symptoms than levodopa, but they carry a lower risk of early dyskinesias. That’s why they’re often used first in younger patients to delay levodopa use. But they can cause side effects like sleepiness, hallucinations, and compulsive behaviors. Levodopa remains the most powerful symptom reliever. The choice depends on age, symptom severity, lifestyle, and personal tolerance.
What’s the best way to manage ‘off’ episodes?
‘Off’ episodes - sudden returns of stiffness, tremor, or freezing - are managed with fast-acting options. Inbrija (inhaled levodopa) works in under 10 minutes and is approved for this purpose. Some patients use fast-dissolving levodopa tablets or subcutaneous infusions. Non-medication strategies include physical movement (like marching in place or rocking side to side) to trigger movement. Keeping a symptom diary helps identify triggers like stress, missed doses, or high-protein meals. Always talk to your neurologist before adding new treatments.





Jade Hovet
December 12, 2025 AT 12:08Lauren Scrima
December 13, 2025 AT 22:14Harriet Wollaston
December 15, 2025 AT 15:49Constantine Vigderman
December 16, 2025 AT 02:58Shelby Ume
December 17, 2025 AT 07:20nina nakamura
December 18, 2025 AT 14:20Willie Onst
December 20, 2025 AT 04:09Jennifer Taylor
December 20, 2025 AT 18:22nithin Kuntumadugu
December 22, 2025 AT 00:09Bruno Janssen
December 22, 2025 AT 10:55sharon soila
December 23, 2025 AT 07:52Tom Zerkoff
December 24, 2025 AT 00:24Yatendra S
December 24, 2025 AT 17:48Ronan Lansbury
December 25, 2025 AT 17:25