In the US, generic drug pricing is a complex system where 90% of prescriptions are filled with generics, yet patients still face unexpected costs. Unlike countries with direct price controls, the US relies on market competition and intricate regulations that often leave consumers confused. Understanding how government policies shape these prices can help you navigate costs and make informed choices.
How the US System Works
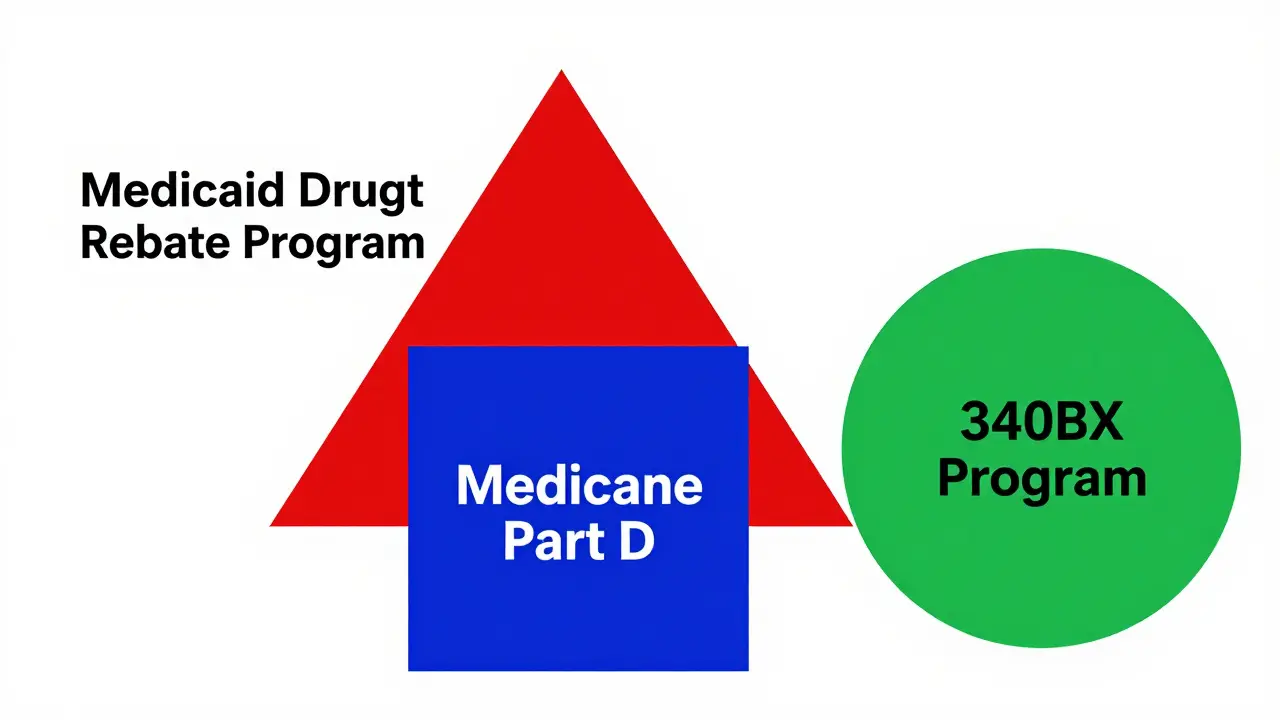
The core of US generic drug pricing is the Medicaid Drug Rebate Program (MDRP) a federal requirement for drug manufacturers to provide rebates for Medicaid-covered medications. Established in 1990, it calculates rebates as the greater of 23.1% of the Average Manufacturer Price (AMP) or the difference between AMP and the "best price" offered to commercial buyers. CMS updates these figures quarterly. In 2024, MDRP generated $14.3 billion in rebates-78% of all Medicaid drug rebates. This system lowers Medicaid costs but doesn’t directly affect what you pay at the pharmacy.
For Medicare Part D beneficiaries, out-of-pocket costs vary significantly. Low-Income Subsidy (LIS) recipients pay $0-$4.90 per generic prescription in 2025, while others face 25% coinsurance during initial coverage. The Inflation Reduction Act (IRA) a 2022 law that reformed Medicare drug pricing capped annual out-of-pocket spending at $2,000 starting in 2025. A patient taking multiple generics saw their yearly costs drop from $412 in 2022 to $327 in 2024, according to CMS data.
Another key mechanism is the 340B Drug Pricing Program a federal program requiring discounted drug sales to safety-net clinics. It mandates manufacturers sell drugs at 20-50% below AMP to clinics serving vulnerable populations. Community Health Center Association data shows 87% of these clinics report better patient adherence due to lower costs. However, this program doesn’t apply to most commercial patients.
How the US Compares to Other Countries
Unlike the US, countries like Canada, the UK, and Germany use direct price controls. The UK’s National Institute for Health and Care Excellence (NICE) a government body that negotiates drug prices sets prices through formal negotiations. Germany uses a benefit assessment system to evaluate cost-effectiveness before pricing. In 2025, IQVIA found US generic prices were 1.3 times higher than the OECD average. For example, generic metformin costs $1.50 per pill in Germany but $2.50 in the US.
Yet the US system has advantages: 90% of prescriptions are generics by volume, compared to 65% in Europe. This is because the US gets generics to market faster after patent expiration. However, this speed comes with volatility. When only two manufacturers make a drug-like pyrimethamine (Daraprim)-prices can spike 300%. In 2024, Daraprim’s cost jumped from $20 to $80 per pill due to limited competition.
Real-World Patient Impacts
Reddit user "PharmTech2020," a pharmacy technician, shared in October 2025: "I see Medicare Part D patients pay $0 to $45 for generics depending on their plan’s formulary tier." A 2025 KFF survey found 30% of Americans struggle to afford medications, with generic costs affecting 18% of respondents. Medicare beneficiaries using generics reported average out-of-pocket costs of $327 annually in 2024-down from $412 in 2022 due to IRA reforms.
Consider Mary Johnson, a 68-year-old Florida retiree. She pays $15 monthly for generic lisinopril through Medicare Part D but once faced a $90 bill when her pharmacy switched to a different generic manufacturer with higher copay requirements. A September 2025 Consumer Reports investigation found 22% of patients experienced unexpected generic drug costs exceeding $50 per month. Senate HELP Committee data also revealed 68% of generic drug "savings" from rebates never reach consumers due to opaque Pharmacy Benefit Manager (PBM) practices.
Current Policy Shifts (2025-2026)
In September 2025, Pfizer agreed to accelerate generic competition for three high-cost drugs, potentially saving $4.2 billion annually. CMS finalized 2026 Medicare Part D parameters in August 2025, lowering the generic drug deductible from $595 to $545. The most significant change involves Medicare’s second round of drug price negotiations, with CMS selecting 15 Part D drugs for 2027, including generic versions of apixaban (Eliquis) and rivaroxaban (Xarelto). These drugs account for $40.7 billion in spending with 5.3 million Medicare beneficiaries.
Evaluate Pharma predicts these negotiations could reduce prices by 25-35% starting in 2027. The Congressional Budget Office estimates IRA provisions will save $196 billion in Medicare spending over 2024-2033, with generic drug provisions contributing $12.7 billion. However, legal challenges continue-PhRMA sued in May 2025 over the Most-Favored-Nation pricing executive order, arguing it violates constitutional protections.

Expert Opinions on Regulation
Opinions on government control of generic prices are split. The Academy of Managed Care Pharmacy (AMCP) explicitly opposes regulation, stating in their 2025 position paper that it creates "unintended consequences" and advocating for "elimination of barriers to competition." Dr. Mark McClellan, former FDA Commissioner, argued in a March 2025 JAMA article that "policy solutions should enhance competition to lower drug prices, benchmark reimbursements to efficacy, and address supply chain elements."
Conversely, Dr. Peter Bach of Memorial Sloan Kettering testified before the Senate Finance Committee in February 2025 that "the US pays 138% more for generic drugs than other high-income countries due to fragmented purchasing power," advocating for Medicare to negotiate prices like the VA system, which achieves 40-60% discounts through centralized purchasing. The CBO estimated expanding Medicare negotiation to select generics could save $12.7 billion over ten years, though this represents only 0.3% of total prescription drug spending. Industry analysts like David Epstein warn that excessive controls could reduce innovation in drug manufacturing processes.
What Patients Can Do
Here are practical steps to manage generic drug costs:
- Use the Medicare Plan Finder a tool to compare Part D plans-48 million users accessed it in 2024 to find plans with $0 premium generics.
- Check if your pharmacy participates in the 340B Drug Pricing Program for discounted drugs at safety-net clinics. Community Health Center data shows 87% of clinics report improved adherence due to lower costs.
- Ask about generic substitution rules: 49 states allow automatic substitution but with varying criteria. If you travel frequently, verify your pharmacy’s policies to avoid unexpected copays.
- Request a review of your Part D formulary tier. Some plans charge higher copays for specific generic manufacturers even if the drug is chemically identical.
- Monitor PBMs: Since 68% of generic "savings" from rebates never reach consumers, ask your pharmacy to explain how rebates affect your out-of-pocket costs.
For example, a 2025 AARP report found the average Medicare beneficiary spends 4.7 hours annually understanding Part D coverage details. LIS beneficiaries require less time (2.3 hours) due to simplified cost structures. State health insurance assistance programs fielded 12.7 million inquiries in 2024-mostly about generic drug costs. Don’t hesitate to use these free resources to clarify your coverage.





Rene Krikhaar
February 6, 2026 AT 06:15I've seen patients struggle with costs even though generics are supposed to be cheaper.
The Medicaid rebate system is complex but important.
Thanks for breaking it down.
We need more transparency.
The current system is confusing for many.
PBMs play a big role in pricing.
Many patients don't understand how their copays are calculated.
The 340B program helps clinics but isn't available to everyone.
Medicare Part D has different tiers.
Some plans have higher costs for certain generics.
It's important to review your plan annually.
The Inflation Reduction Act has helped reduce out-of-pocket costs.
But there's still room for improvement.
Patients should use resources like Medicare Plan Finder.
State health insurance programs can help clarify coverage.
Overall, knowledge is key to managing costs.
one hamzah
February 6, 2026 AT 08:22PBMs are tricky but we need to fix them
#HealthcareForAll 😊
Matthew Morales
February 7, 2026 AT 15:47Pharmarcy Benefit Managers are tricky but this info helps 😊
Diana Phe
February 9, 2026 AT 02:57Carl Crista
February 10, 2026 AT 03:50Andre Shaw
February 10, 2026 AT 11:58The real issue is not government control but lack of competition
These 'generic' drugs are overpriced because of monopolies
We need to break up the PBMs
This post is way too soft on the system
Dr. Sara Harowitz
February 11, 2026 AT 07:15Kieran Griffiths
February 13, 2026 AT 03:15Here's a tip: always check your Part D plan's formulary tier
Small changes can save you hundreds
Stay informed and advocate for yourself
You've got this!